12+ Which Intervals Are Affected By The Addition Of A Catalyst
How are the following aspects of a reaction affected by the addition of a catalyst. Aa catalyst Ban indicator Celectrical energy Dthermal energy 5The activation energy of a chemical reaction can be.

Improving Geriatric Care And Reducing Hospitalisations In Regional And Remote Areas The Benefits Of Telehealth Louise Lillicrap Christine Hunter Peter Goldswain 2021
7 _ÿcŒOšzS 3 1 and 3 4 3 and 4 1 1 and 2 2 2 and 5.

. The addition of a catalyst will A shift the equilibrium to the. A catalyst changes the Gibbs energy G of the reaction and equilibrium constant of the reaction. A catalyst lowers the activation energy for example by providing a surface for the reaction to occur on.
When one mole of a certain compound is formed from its elements under standard conditions it absorbs 85 kiloJoules of heat. At equilibrium the rate of forward reaction is equal to the rate of backward reaction. 1 Both potential energy and average kinetic energy increase.
Rate of reaction can be increased by using a catalyst. Which intervals are affected by the addition of a catalyst. Catalysts speed up chemical reactions.
2 Both potential energy and average. How are the following aspects of a reaction affected by the addition of a catalyst. Suppose you add a catalyst to the system already at equilibrium nothing happens as the.
Does the addition of a catalyst affect the change in enthalpy of a reaction. A I and 2 C 2 and4 B land 3 D 3 and 4. Which intervals are affected by the addition of a catalyst.
Which intervals are affected by the addition of a catalyst. From this relation it can be said that the addition of the catalyst affects the rate of reaction and this inverse effect on the activation energy. Only very minute quantities of the catalyst are required to produce a dramatic change in the rate of the reaction.
This is known as the Arrhenius equation. Given the potential energy diagram for a chemical reaction. Which statement describes the energy of the particles in this sample during interval DE.
Meanwhile the concept of catalysis was first researched by chemist Elizabeth Fulhame and it. A catalyst will affect the rate of the forward reaction by changing the. Catalyst is a term derived from Greek καταλύειν meaning to annul or to unite or to pick up.
This is really because the reaction. Which numbered interval will change with the addition of a catalyst to the system. A correct conclusion from this statement is that the.

Paediatric Dept Annual Report 2012 By Department Of Paediatrics University Of Calgary Issuu
Full Article Peer Reviewed Abstracts

Uio 66 Ce Metal Organic Framework As A Highly Active And Selective Catalyst For The Aerobic Oxidation Of Benzyl Amines Sciencedirect

Us20150080546a1 Production And Use Of 3 4 And 4 4 Dimethylbiphenyl Isomers Google Patents

Supramolecular Catalysis Of Acyl Transfer Within Zinc Porphyrin Based Metal Organic Cages Inorganic Chemistry
Regents Chemistry Exam Explanations January 2016

Pdf Chem F3 Q Ans Full Amour Rashid Academia Edu

Solid Lewis Acid Base Pair Catalysts Constructed By Regulations On Defects Of Uio 66 For The Catalytic Hydrogenation Of Cinnamaldehyde Sciencedirect

Synthesis And Crystal Structure Of A New Copper Ii Complex Designed To Produce Efficient Successor Of Cu2o Toward Synergy Of Adsorption And Photodegradation Of Mb Razmara 2020 Applied Organometallic
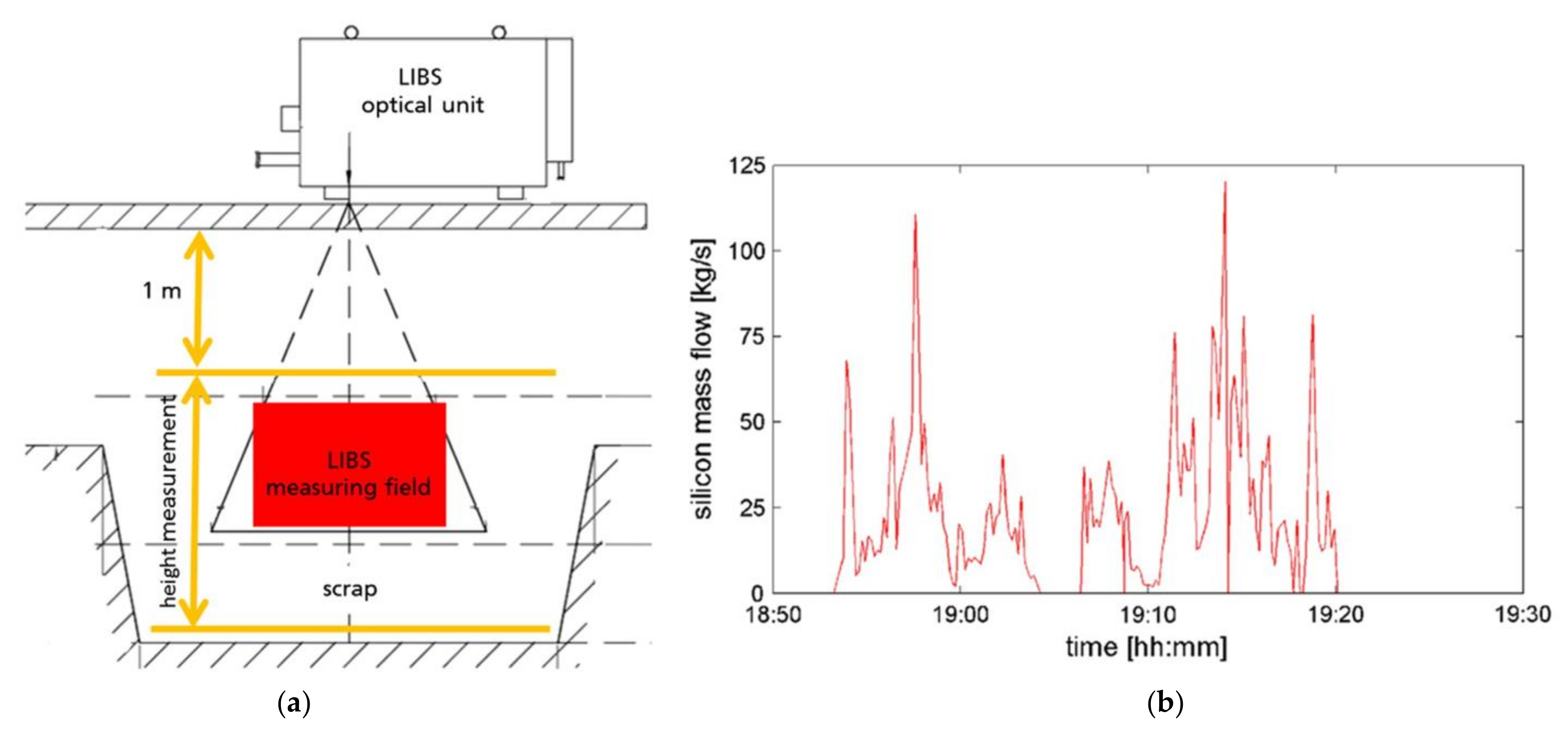
Applied Sciences Free Full Text Review Of Element Analysis Of Industrial Materials By In Line Laser Induced Breakdown Spectroscopy Libs

Quiz 4 Potential Energy Diagrams

Programme Book Flip Ebook Pages 51 100 Anyflip

Ai59pqhbk1u3vm
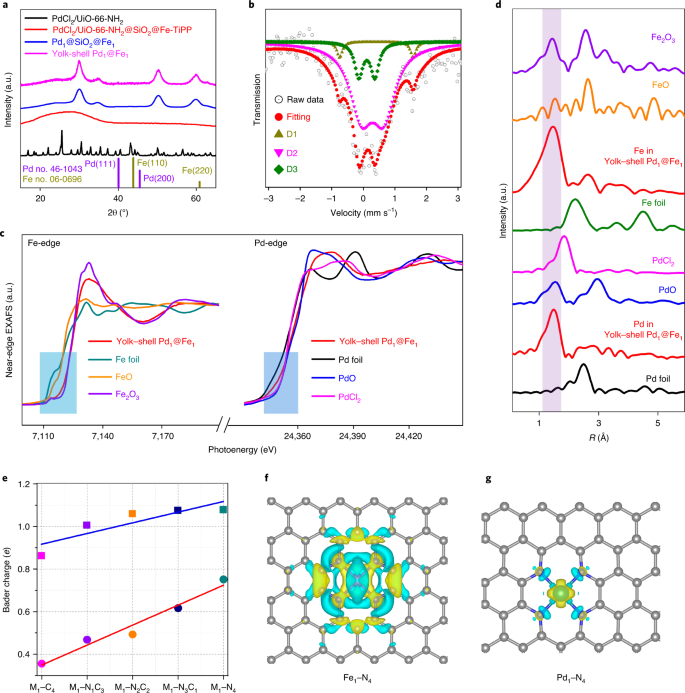
Simultaneous Oxidative And Reductive Reactions In One System By Atomic Design Nature Catalysis
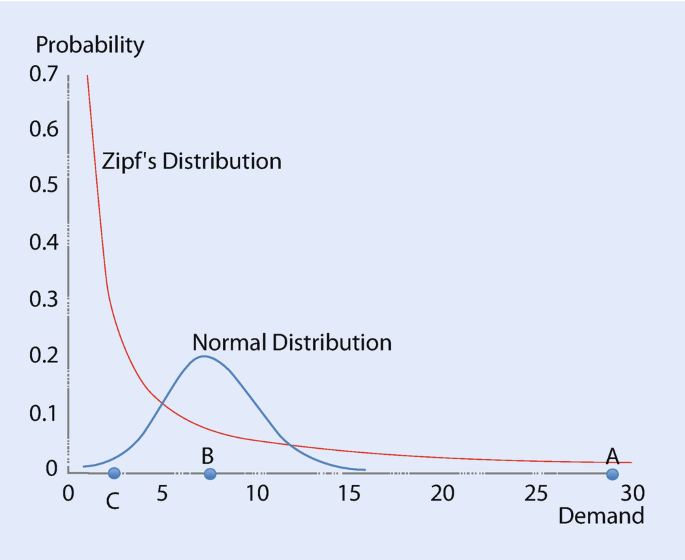
Demand And Market Research For Media And Information Products Springerlink

Pdf Morphological Consequences Of Catalytic Hydrogenation Of Polymers In The Bulk Dale W Schaefer Academia Edu

Fsci Ncsr 103121